Abstract
Despite the achievement of hematological remission (CR), acute myeloid leukemia (AML) patients with persistence of detectable disease assessed with high sensitivity techniques (multicolor-flow-cytometry, MFC, or PCR-based molecular analysis) show a poor outcome. The aim of our study was to evaluate the prognostic impact of MFC and molecular minimal residual disease (MRD) assessment in a cohort of AML patients who had received a fludarabine-high dose cytarabine containing induction (FLAI) as front-line treatment by identifying optimal time-points (TPs) and MFC and molecular MRD cut-off values.
One hundred and ten consecutive AML patients treated in our center between 2004 and 2014 were retrospectively analyzed. Median age was 47 years. Median follow up was 59 months. MRD was assessed at 3 TPs during treatment; TP1, after induction I; TP2, after induction II; TP3, after consolidation therapy for patients who did not undergo hematopoietic stem cells transplantation (HSCT) or prior transplantation.
Two thresholds of MFC-MRD were evaluated; MFC-MRD undetectable (MFC-MRD <0.025%) and low MFC-MRD burden (MFC-MRD <0.1%). A sub-analysis for patients carrying NPM1 mutation was performed analyzing NPM1 expression levels on bone marrow (BM) samples . A reduction >3.5 log of NPM1 transcript at TP1 was considered optimal as per our published experience.
For patients presenting WT1 over-expression at diagnosis WT1-MRD was evaluated at TP1, with a cut-off of for negativity of WT1 copies/104 ABL lower than 250 on BM samples.
Following FLAI, 103/120 patients achieved CR (86%). Out of 103 patients in CR after FLAI MFC-MRD data were available for 88 patients at TP1 (85%) and for 77 at TP2 and TP3 (75%). At TP1, 30/88 (34%) patients had undetectable disease. This percentage remained substantially unchanged at TP2. Patients with MFC-MRD <0.1% were 41/88 (47%) at TP1; this percentage increased to 62% at TP2. At TP3 the percentage of patients with undetectable MFC-MRD or with MFC-MRD <0.1% was comparable to what was observed at TP2.
After a median follow-up of 60 months, out of 103 CR patients 26 relapsed. Patients undergoing HSCT in first CR were censored at the time of transplant for CI of relapse evaluation.
In competitive risk analysis, the 3-year CI of relapse was 42.2%. In univariate analysis a higher CI of relapse was significantly associated with secondary disease, high risk group according to ELN 2017, time from hematological recovery following induction 1 to start of induction 2 (i.e. >20 days), detectable MFC-MRD and MFC-MRD ≥0.1% both at TP1 and TP2 and positive WT1-MRD at TP1.
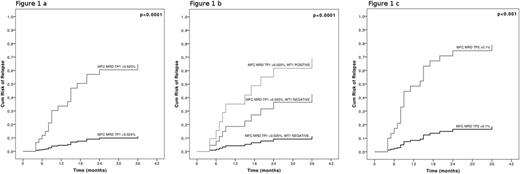
Multivariate analysis revealed MFC-MRD ≥0.025% at TP1 as the strongest factor affecting the CI of relapse (p<0.0001, Fig. 1a). Interestingly, the combination of MFC-MRD with the most stringent sensitivity cut off (0.025%) and WT1-MRD at TP1 led to the identification of 3 groups of patients having a significantly different CI of relapse. Patients for whom both methods gave positive results displayed the worst outcome (p<0.0001, Fig. 1b). Patients with MFC-MRD ≥0.1% at TP2 had the highest CI of relapse (p<0.001, Fig. 1c).
Patients with undetectable MFC-MRD at TP1 had the best 3-year overall survival (OS) (92.5%, p< 0.001). Multivariate analysis showed that risk group according to ELN 2017 and MFC MRD ≥0.1% at TP1 were the only factors that significantly impacted on OS.
Our study included 42 AML with mutated NPM1. Thirty-five achieved CR and had available NPM MRD data. At TP1 18/35 (51%) patients achieved >3.5 log reduction of NPM1 transcript. At TP2 27/35 (77%) patients obtained NPM1 negativity. Three-year OS for patients showing more or less than 3.5 log transcript reduction at TP1 was 94.1% vs 48.6%, respectively (p<0.03). Three-year OS for patients achieving NPM-MRD negativity at TP2 was 90.5% compared to 21.4% for those who remained positive (p<0.001). Multivariate analysis showed that >3.5 log reduction of NPM- MRD at TP1 was the strongest predictor for OS (p<0.05).
Our study is the first report analyzing the prognostic impact of longitudinal MRD assessment for AML patients receiving a fludarabine-containing induction regimen. Early (post induction) MRD assessment is the strongest factor affecting CI of relapse and survival. This observation is probably linked to the strong anti-leukemic activity of FLAI and may allow planning of alternative therapeutic interventions in patients with suboptimal response.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal